Comprehension
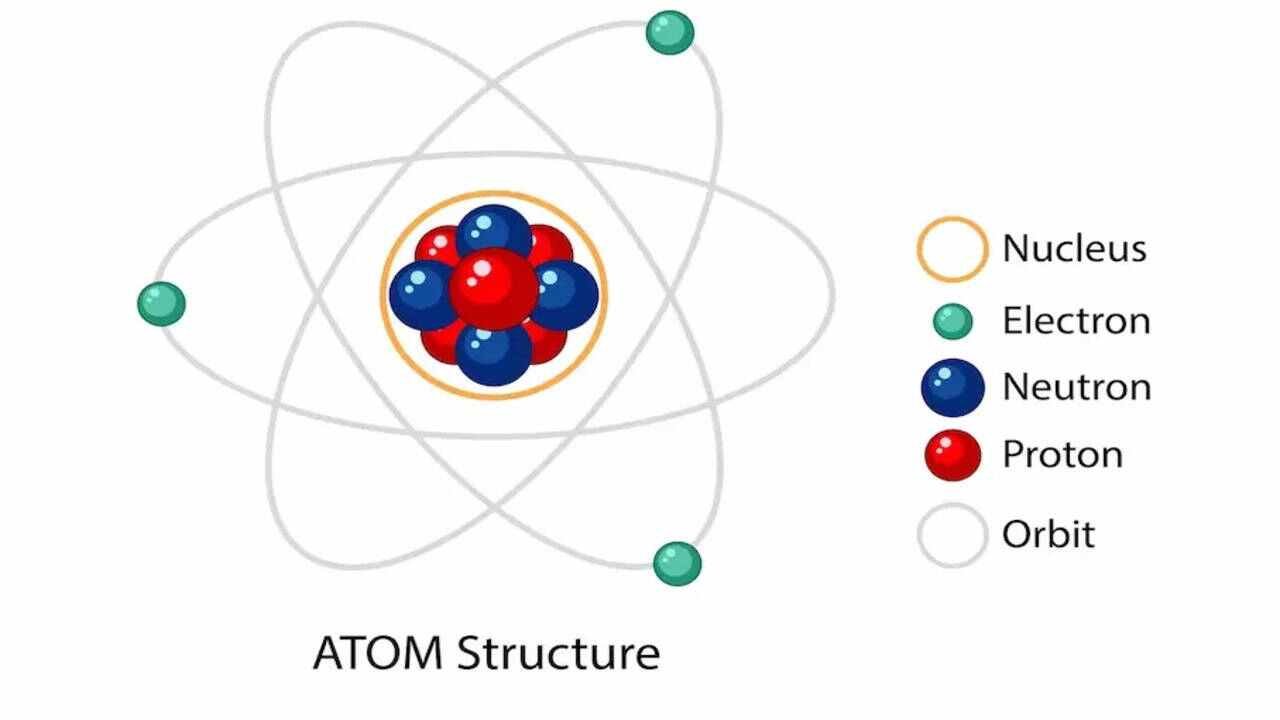
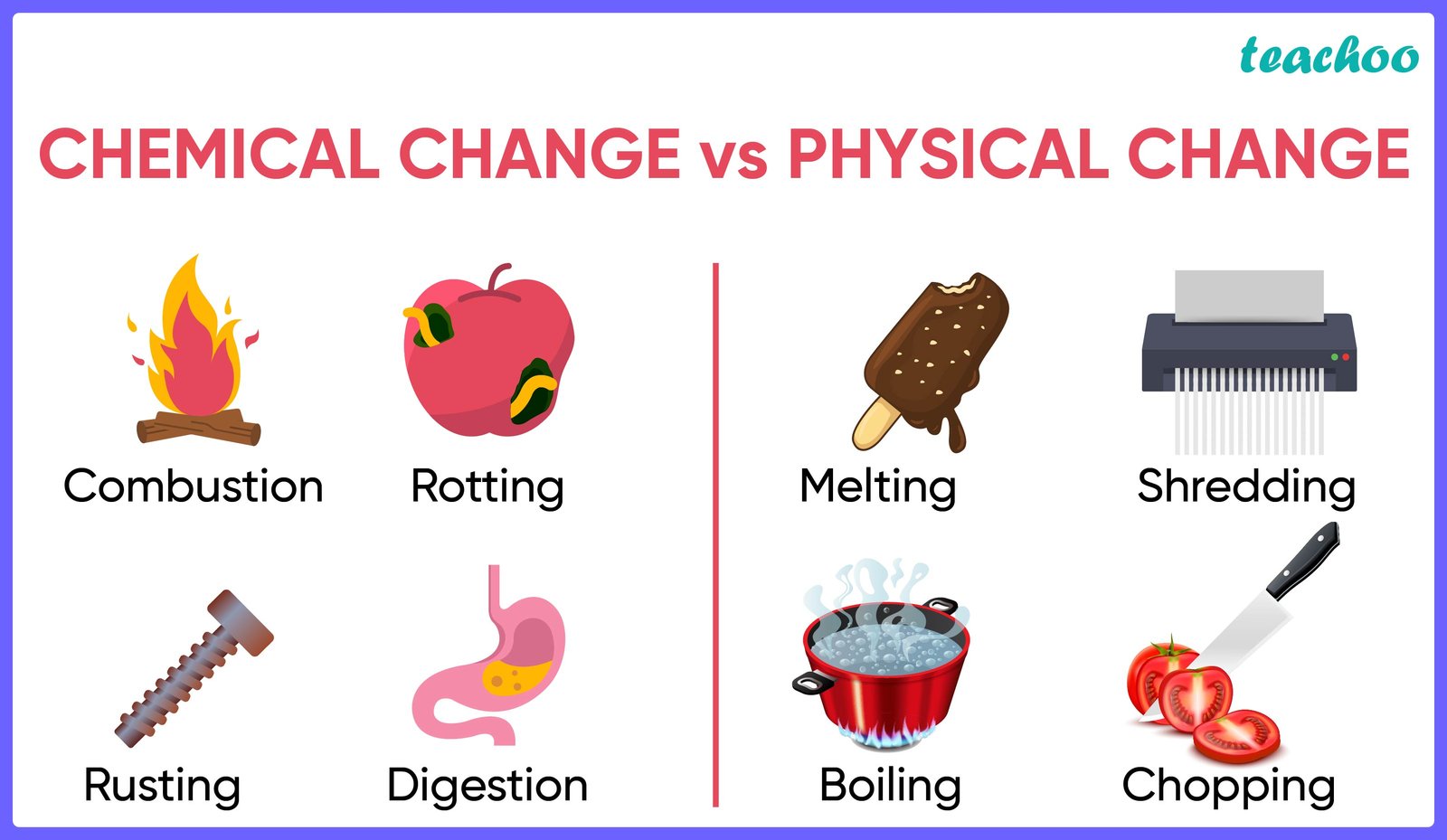
Physical changes and chemical changes are two
fundamental types of changes that matter can undergo. A physical change affects
one or more physical properties of a substance without altering its chemical
composition. For example, melting ice into water is a physical change because
the substance remains H₂O, just in a different
state. Other examples include breaking a glass or dissolving sugar in water. Physical
changes are usually reversible.
On the other hand, a chemical change results in the
formation of one or more new substances with different properties. This occurs
when chemical bonds between atoms are broken and new ones are formed. For
example, burning wood is a chemical change because it transforms the wood into
ash, carbon dioxide, and water vapor, which are entirely different substances.
Chemical changes are often irreversible, such as when an egg cooks or when iron
rusts.